Stomatogastric Ganglion
a. Activity patterns: The stomatogastric ganglion (STG) of
crustaceans controls and coordinates the activity of around 40
pairs of striated
muscles of the stomach.
It possesses around 30 reidentifiable neurons [F2] which are mostly
motorneurons. These produce two primary activity rhythms of motor output. The
pyloric rhythm causes coordinated sequences of contraction
in muscles controlling the
pylorus (operating hair-plates). The
gastric rhythm controls muscles of the
gastric mill (operating the gastric
teeth). The ganglion can be excised from the animal along
with its sensory, motor, and central nerve connections and kept alive in a
Petri dish
under physiological saline. Fine wires placed in contact with
the nerves and insulated from the saline with vaseline pick up
electrical nerve impulse signals travelling out the nerves to the
severed muscle connections. Axons to different muscles discharge
bursts of impulses which correspond well to the EMG patterns in
more intact preparations. The
pyloric pattern
is characterized by a three-part cycle, in which a burst of impulses
in the network driver neurons (PD/AB) is followed by a burst in
the motorneuron to the antagonist muscle (LP) and finally a barrage of impulses
to more posterior muscles controlling pyloric sieve plates (PE/PL). The
gastric pattern
consists of alternating bursts in two semi-independent antagonistically
arranged motor groups, one controlling the lateral teeth
and one controlling the medial tooth.
b. Synaptic connections: The different motor patterns
observed can be explained partly by the synaptic connections and
properties among stomatogastric neurons and partly by intrinsic
properties of the neurons themselves. Both chemical and
electrical connections are found. Most chemical connections
within the ganglion ("intrinsic" connections) are inhibitory. In the
pyloric system the driver neurons (PD/AB) put strong
inhibitory synapses on the remaining "follower" neurons so that
during driver activity, followers are shut off. After driver
bursts terminate spontaneously, followers are free to fire in a
sequence which is determined partly by their synaptic connections
and partly by their cellular properties. In the
gastric system, antagonistic neurons of the lateral
tooth set are reciprocally inhibitorily interconnected, while the driver
neurons of the medial tooth (return stroke) inhibit their
antagonists, but not the reverse. The synaptic wiring of the
gastric system is substantially more complex than that of the
pyloric.
c. Cellular properties: In addition to fairly conventional
repetitive firing properties, stomatogastric neurons
possess three special cellular properties which significantly
shape their activity patterns. A delaying mechanism is
activated in some cells upon release from a hyperpolarization, and
retards the recovery of the cell from previous synaptic
inhibition. A post-inhibitory rebound [F11] mechanism is
activated in some cells upon release from a hyperpolarization and
promotes rapid recovery and vigorous firing following release
from previous synaptic inhibition. A plateau mechanism [F12]
endows many cells with an endogenous burst-promoting property
which can lead to endogenous repetitive bursting in some cases
(in driver cells) or regenerative burst triggering and
suppression in others.
Additional information:
calcium currents
d. Central control: The ganglion is under the control of
higher centers. Almost all bursting activity ceases when input
from these centers is blocked. The activity can be restored by
stimulating input nerves [F13] electrically. The key mechanism
by which this control is exerted is through the modulatory
regulation of the plateau mechanism. Induction of plateau
properties [F14] in specific STG target neurons can occur when
specific inputs are stimulated, or when they are exposed to
specific neuromodulator substances (presumably) contained in
those input neurons. Modulatory control of synaptic strength is
also found in the system.
e. Cellular morphology: Motorneurons of the stomatogastric
ganglion are monopolar cells with a cell body located on the
outer surface of the ganglion, a main neurite traveling through
the neuropile in a typically contorted path and finally exiting
as an axon projecting to target muscle(s). For a distance of
a few hundred microns along the main neurite, secondary neurites
are given off which branch repeatedly into the ganglionic
neuropile. The soma is "inexcitable" as far as impulses are
concerned, but it shows strong rectification and in some cells
exhibits a delaying mechanism. Nerve impulses appear to be
confined to the axons. Synaptic inputs and outputs occur on the
finer distal branches of the cell, and plateau properties are
assumed to reside close to synaptic input sites (given their
dependence on modulatory inputs).
Additional information: Hartline-Graubard-Wilensky 2005 (unpublished manuscript)
f. Synaptic properties: Electrotonic synapses [F15] tend
to occur between synergistic neurons, but this is not universally
the case. Rectifying electrotonic connections are common, and
modulation of the strength of electrotonic connections by
neuromodulators has been reported. Chemical synapses are of two
types, spike mediated (phasic) and tonic. Glutamatergic
chemical synapses produce rapidly-rising IPSPs in target neurons,
while cholinergic IPSPs are much slower to rise. The difference
may promote phase shifts and different roles for otherwise
similar presynaptic neurons. Chemotonic interactions [F17]
probably employ the same release sites and mechanisms as do
phasic PSPs, but they are activated by slow membrane voltage
changes and do not require spiking in presynaptic neurons. They
are quite strong and can coordinate the modulator-induced
oscillations of the STG in the presence of tetrodotoxin (TTX),
which eliminates all spikes.
g. Neurotransmitters and neuromodulators: A large number
of substances is found with neuromodulatory action on typically
very specific groups of cells within the stomatogastric nervous
system. The neuromodulators undoubtedly act via one or more of
the conventianal second messenger systems, but to date studies on
this have been limited.
Additional information: cAMP studies,
cGMP studies.
h. Modeling.
i. Pyloric network
j. Gastric network
STNS
a. Coupled networks
b. Sensory components
c. Anatomy
d. Behavior
e. Neuromuscular physiology
f. Comparative and evolutionary aspects
[F1]
Anatomy of the spiny lobster stomach
(Panulirus)
The figure below shows a diagram of the stomach of a spiny lobster (Panulirus) with
muscles color coded according to phase of contraction in the motor patterns (labels with
lower case letters and numbers), nerves (heavy black lines) and ganglia of the stomatogastric
nervous system (STG = stomatogastric ganglion; OG=oesophageal ganglion; CG=commissural ganglion).

(source:
Hartline and Maynard 1975 Fig. 1)
Pyloric pattern
(Panulirus)
The "pyloric" pattern of motor activity in the intact spiny lobster (Panulirus
involves a three-part cycle as shown in the
electromyogram (EMG) recordings below. This activity controls a
set of sieve plates and valves in the posterior region of the
stomach. It apparently sieves food particles and may mix them
with digestive secretions.

(source:
Hartline and Maynard 1975 Fig. 5A)
Pyloric muscle sequence
(Panulirus)
The muscles active at each phase of the pyloric cycle are shown
in the diagram below. Activity of the pyloric dilator muscles
mediated by the two PD neurons appears to open a valve to the
pyloric region which is then closed in the second phase by an
antagonist, the lateral pyloric muscle operated by the LP cell.
In the third phase, a sheet of pyloric muscles contracts under
the activation of several PY neurons (divisible into PE and PL
subtypes) giving overall a peristaltic appearance to the pyloric
surface. The two muscles located on the anterior (cardiac)
portion of the stomach control a curious valve structure, the
operation of which is described in Squilla (ref: Tazaki)

(source:
Hartline and Maynard, 1975 Fig 4)
Gastric pattern
(Panulirus)
The gastric motor pattern involves two coupled muscle-contraction
sequences that operate the gastric mill. EMGs from these muscles
are shown in the figure below. Alternation between closer and
opener muscles for the lateral teeth is controlled by the LC/GP -
LG motorneurons [also called LG/MG - LPG]
*. Alternation between protractor and
retractor muscles for the medial tooth is controlled by GM - CP [also called DG]
*.

(source:
Hartline and Maynard, 1975Fig.3A)
Gastric mill muscle sequence
(Panulirus)
Loose coupling between the two tooth groups generates a coordinated pattern
such that GM firing commences usually somewhat after the LC/GP
* firing. Thus the
power stroke (closer activity) of the lateral teeth occurs
somewhat before that of the medial tooth (protraction), and the
reverse sequence of return stroke muscle activity is similarly
phase shifted, as shown in the diagram below.

(Source:
Hartline and Maynard, 1975, Fig.4)
Gastric mill operation
(Panulirus)
The diagram below shows the two sets of gastric mill teeth, the
lateral teeth (top) and the medial tooth (below) along with the
primary muscles operating them. The contraction sequence
described above and indicated by bars below the tooth diagrams
apparently clamps food in the lateral teeth, whereupon the medial
tooth rasps down and forward over the immobilized food, helping
to macerate it.

(Source:
Hartline and Maynard, 1975, Fig.2)
Stomatogastric nervous system in a dish
(Panulirus)
The diagram below shows the various input and output nerves to
the stomatogastric ganglion (STG) as seen in a typical "combined"
preparation (see Russell 1976). Nerves going to single muscles
or small groups of muscles may be individually followed, freed,
and recorded from. The left and right commissural ganglia (CG)
can be removed along with segments of the paired
circumoesophageal commissures. From these ganglia, two nerves,
the inferior (Ion) and superior (Son) oesophageal nerves diverge
and then reconverge at the oesophageal ganglion (OG), forming the
stomatogastric nerve (Stn), the primary input to the STG.
Specific reidentifiable input axons run in these different
nerves.

Nerve impulse discharge patterns from pyloric neurons
(Panulirus)
The same coordinated patterns of pyloric activity can be recorded
in excised ganglia from electrodes placed on the motor nerves
traveling from the ganglion to the muscle. In this recording,
connections to the CNS have been left intact and the ganglion is
cycling spontaneously. The pattern consists of sequential bursts
in three driver neurons (2 PD motorneurons and one AB
interneuron), then LP and IC, and finally PE and PL (=PY). The
VD burst typically precedes PD/AB and may overlap it.

(source:
Hartline et al.1988, Fig.6)
See Scott Hooper's web page
for an update on recent studies of phase maintenance and pattern generation in the pyloric network.
Nerve impulse patterns from gastric neurons
(Panulirus)
In the absence of connections to the CNS, the gastric motor
pattern is completely absent. In the recording below, a
"command" fiber" in the input nerve (stomatogastric nerve) is
being stimulated at low frequency (ca 2 Hz), which restores
active cycling to the gastric network.

(Source:
Russell and Hartline, 1984, Fig.2)
Pyloric circuit wiring
(simplified; Panulirus)
Intracellular recordings from pyloric somata reveal a variety of
subthreshold events. Particularly evident are inhibitory
synaptic potentials which can be matched 1:1 with identified
inpulses in other cells. This serves to establish a "wiring
diagram" for the system. The PD/AB cells normally dominate the
network through their strong inhibitory synapses onto all other
pyloric neurons. When the endogenously-generated PD/AB burst
terminates, the other "follower" neurons fire in sequence. In
spiny lobsters (Panulirus), LP activity inhibits PD/AB and also
weakly inhibits the PYs. PL activity strongly inhibits the LP.
Both AB and LP strongly inhibit the VD. In addition, there are
bidirectional electrotonic connections in the PD-AB-VD group and
among PLs, and a rectifying connection from LP to PLs.

(Source:
Russell and Hartline, 1982, Fig.2)
Gastric circuit wiring
(Panulirus; simplified)
Connections among gastric neurons can be determined through
intracellular recordings as they are for the pyloric system. The
reciprocal inhibition between the antagonistic groups of lateral
teeth motorneurons (LC/GP [LG/MG] and LG [LPG] ensures little
overlap in activation of members of the pair. The inhibition
from AM/CP[DG] onto GM helps keep separate the activity of those
two antagonistic groups. The other connections are complex and
how they relate to observed activity is not clear. It was
originally thought that no properties capable of producing
endogenous bursting are present in the gastric net, so that
gastric bursting is an "emergent property" of the synaptic
connectivity. Now it is recognized that a complex of cellular
and synaptic properties, including in some cases endogenous
bursting, are responsible for gastric patterns.
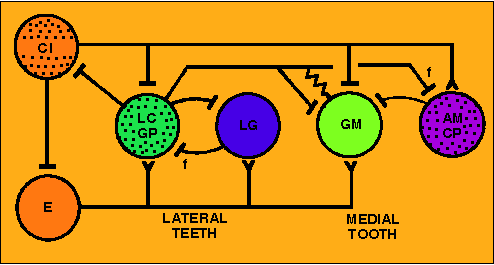
(Source:
Hartline et al.,1988, Fig.1D)
Repetitive firing properties
One reason for the focus of so many research efforts on the
stomatogastric ganglion is that it has so few cells. There is
hope that each cell and each intercellular interaction can be
characterized and its contribution to the network activity
accurately assessed. To make such an assessment, quantitative
measurements must be made of all such properties. The figure
below shows how repetitive firing characteristics of STG neruons
may be measured by fairly conventional means. Firing in response
to a step depolarization is measured as a function of time after
current onset. The frequency adapts along a compound exponential
timne-course with time-constants that are independent of the
strength of the injected current. The numerical values of
parameters characterizing this response can be utilized in
quantitatively accurate computer models to predict the firing
behavior under a variety of other conditions, including natural
ocillations.

(Source:
Hartline and Graubard, 1992, Fig.2.13)
Delaying properties
Some stomatogastric neurons show a pronounced delay in recovery of their excitability
following a brief hyperpolarization, whether synaptically or artificially generated.
In the figure below, two different pyloric follower cells are given equal step excitations
(top trace of each panel) producing the same rate of impulse firing. If the step
excitation is preceded by a hyperpolarizing pre-pulse, there is a delay in the onset of
firing while the cell recovers from the hyperpolarization. As the hyperpolarization is increased
in magnitude (successive sweeps below the control), the delay in the PY cell becomes
disproportionately long compared to that in the LP. A slowly inactivating "A" conductance
appears to underlie this phenomonen. It contributes to the phase lag of PY firing relative to LP
in the pyloric cycle.

(Source:
Hartline, 1979, Fig. 7)
See Scott Hooper's web page
for an update on recent studies of rebound delay in the pyloric network.
Postinhibitory rebound
(PIR)
Certain STG neurons (the LP in the panels below) exhibit a "sag" trajectory
when a sufficiently strong hyperpolarizing current (or synaptic inhibition) is injected
(left panel). The resulting depolarization brings the neuron closer to threshold despite
the maintenance of the suppressing influence. Once the hyperpolarizing input is released,
the cell reaches threshold rapidly, and its firing is augmented in a rebound effect that
enhances temporal contrast in its activity and adds to the strength of its synaptic output.
One paradoxical effect of this mechanism, shown in the panel to the right, is that the TOTAL
number of nerve impulses

(Source:
Hartline and Graubard, 1992, Fig.2.15)
Plateau properties
If an actively bursting stomatogastric neuron is held silent by a small hyperpolarizing current,
it is frequently possible to elicit complete bursts as all-or-nothing responses to brief depolarizing
currents or synaptic inputs. These bursts are underlain by an active depolarizing potential termed a
"plateau potential", by analogy to the plateau phase of a heart muscle actin potential.
Conditions for triggering plateau potentials include a threshold for intensity of the triggering
stimulus which must be exceeded to be successful (upper traces in panel A below). Once triggered,
a plateau can be terminated in a all-or-nothing manner by a hyperpolarizing stimulus of sufficient
magnitude (panel B below). Plateau potentials are turned on and off, sometimes spontaneously and
sometimes by impinging synaptic input, in the normal course of cyclic bursting by the STG. They provide
a major component of the drive that generates burst firing, as well as contributing critically to the
timing of bursts in the stomatogastric system.

(Source:
Hartline et al. 1988, Fig.2)
Activation of pyloric rhythm by input stimulation
In isolated ganglia, both pyloric and gastric activity is at a very low level. The pyloric activity
pattern can be reactivated in the isolated ganglion by supplying "priming" stimulation to
the input (stomatogastric) nerve, as shown in the figure below.

(Source:
Hartline and Maynard, 1975, Fig.5)
Plateau induction

(Source:
Hartline et al. 1988, Fig.5)
Electrotonic interactions
Direct low-pass electrical coupling exists between several of the pairs of STG neurons.
Current injected into one of the members of the pair is reflected as an attenuated voltage
perturbation of the same sign in the coupled cell. In many cases in STG, the coupling is bidirectional:
injection of current in the second cell results in a voltage perturbation of the first. In a few
cases, the connection is "rectifying": current of one sign produces a larger perturbation in the
postjunctional cell than current of the opposite sign. In such cases, the relation is reversed for
current injected into the second cell. The figure below shows the latter case. Hyperpolarizing
current injected into the LP (top trace) produces very little hyperpolarization of the postjunctional
PL, whereas the same magnitude of depolarizing current (which also generates a train of closely
spaced nerve impulses) produces a larger depolarization of the PL. Hyperpolarizing current injected
into the PL (bottom trace) produces a large hyperpolarization of the postjunctional LP. Not only
does depolarizing current to the PL have little effect on the LP, but its activation of strong
chemical synaptic release from PL causes a net hyperpolarization of the LP.

(Source: Graubard and Hartline, unpublished)
Synaptic properties
The figure below shows samples of IPSPs in the pyloric net for
interactions among the three principal pyloric cell types. Note
the substantial difference in shape for PD-produced IPSPs as
compared those produced by AB. PDs are cholinergic, while AB is
glutamatergic (ref: Marder). The numbers beside the synapses in
the diagram represent the strengths of the particular synapses
measured as the number of impulses a single IPSP is capable of
deleting from an on-going train in the postsynaptic cell.

(source: Hartline and Gassie, 1979)
Additional information: inhibitory glutamate currents
Chemotonic properties

(Source:)
blank
Title

(source: , Fig. )
(To be continued ...)
 Back to STG Home Page.
Back to STG Home Page.




















